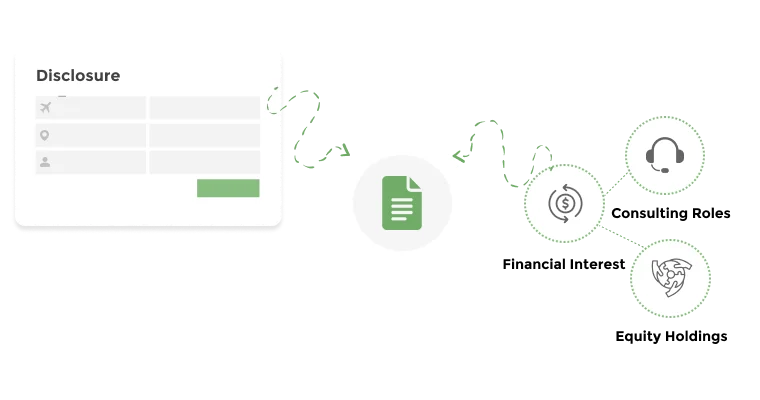
Centralize all financial and non-financial interest disclosures.
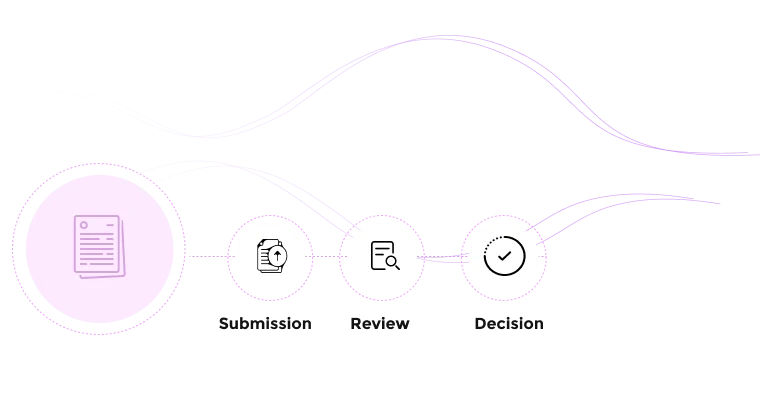
Simplify assessment workflows for administrators and reviewers.
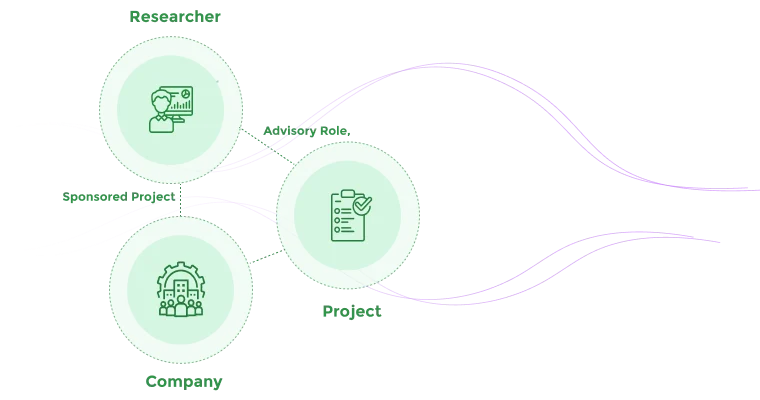
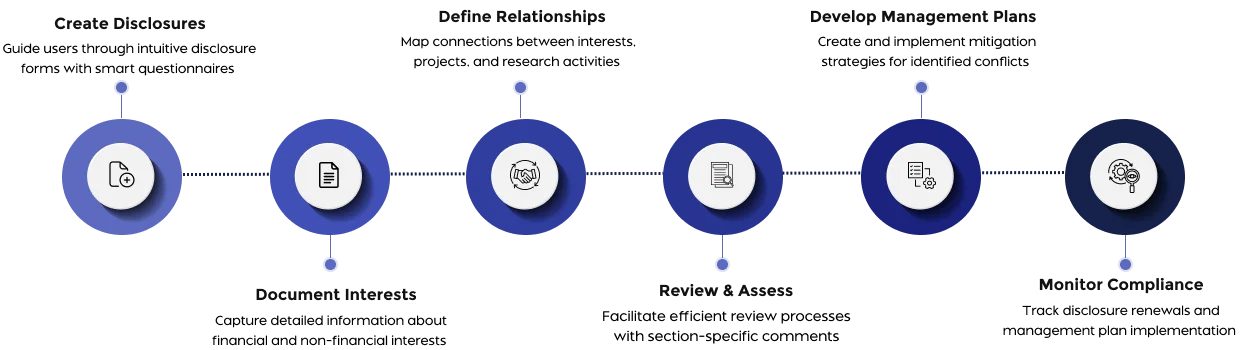
Map connections between researchers, projects, sponsors, and disclosed interests
Automatically assess risk levels using configurable evaluation criteria.
Ensure adherence to federal regulations and institutional policies.
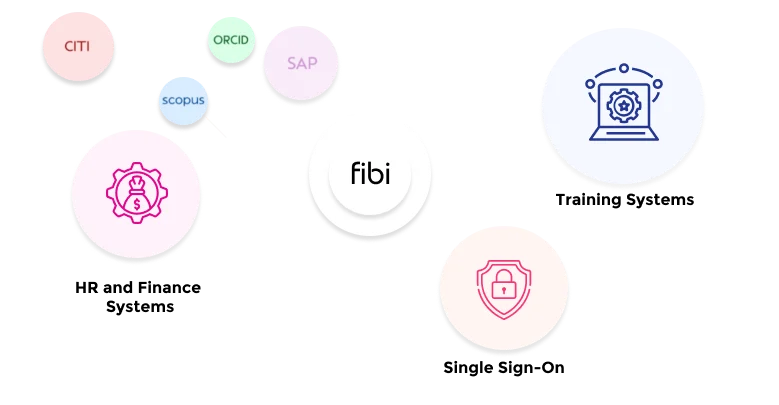
Seamlessly connect with other Fibi modules for end-to-end research oversight.

Support for Financial Conflict of Interest (FCOI), Outside Professional Activities (OPA), and Travel disclosures.

Guide users through disclosure requirements with customizable forms.

Document and manage financial interests with detailed relationship mapping.
Secure electronic certification with comprehensive audit trails.
Track disclosure expiration dates with configurable renewal periods.

Compare disclosure versions and review section by section.

Administrator capability to reassess and modify conflict determinations.

Configurable risk evaluation based on institutional criteria.

Add internal or public reviewer notes with visibility controls.

Assign reviewers dynamically based on role, expertise, or workload.

Centralized database of all disclosed
entities.
Document connections between entities, people, and projects.

Administrative validation of entity
information.
Document entity geographical information for compliance purposes.

Easily view all disclosures linked to a single entity.
Create and manage mitigation strategies for identified conflicts.

Upload supporting documentation for management plans.
Monitor compliance with management plan requirements.

Maintain a full audit trail of all plan versions and updates.

Enhanced lifecycle management
capabilities.
Customized views for reporters,
reviewers, and administrators.
Monitor disclosure status and pending
actions.
Personalized alerts for deadlines and
action items.
Find disclosures by person, department, entity, status, or date.

Generate reports on disclosures, conflicts, and management plans.
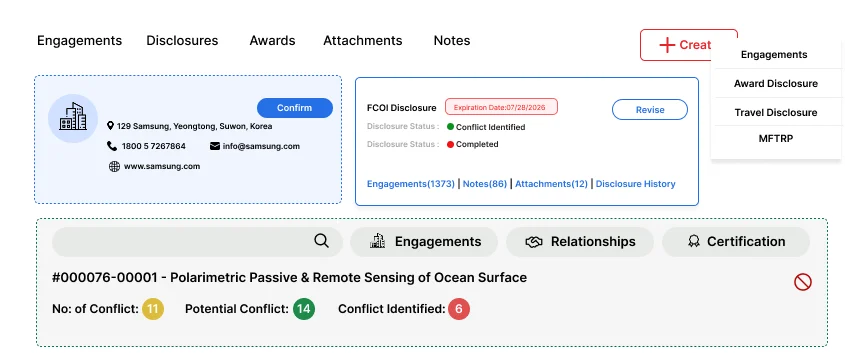
Fibi COI is a centralized solution for managing conflict of interest disclosures in research. It streamlines the entire process of collecting, reviewing, and reporting both financial and non-financial interests. By centralizing all disclosures, Fibi ensures that institutions uphold research integrity and comply with regulations.
Fibi COI automates much of the disclosure workflow. Users fill out customizable screening questionnaires, and the system guides them through what to declare. Administrators and reviewers then use built-in tools to assess disclosures: the review interface lets them see each section of a disclosure and add notes or override conflict statuses if needed. This streamlined process replaces manual paper forms and emails with structured, auditable workflows.
Fibi supports a wide range of disclosures. It explicitly handles Financial Conflict of Interest (FCOI), Outside Professional Activities (OPA), travel disclosures, MFTRP and other disclosures or declarations. In practice, this means faculties can disclose consulting income, equity in companies, or any paid travel, all within the same COI module. The system can be configured to fit an institution’s specific disclosure categories.
Absolutely. Fibi COI is part of the larger Fibi ecosystem and offers seamless integration. It connects with the Grant, IRB, and IACUC modules so that, for example, project data or investigator information is shared across systems. This ensures that when a new grant application is created in Fibi Grants, any relevant COI disclosures for key personnel are immediately visible to administrators.
Yes. Fibi offers flexible deployment options to suit institutional needs. You can choose cloud hosting for scalability and ease of maintenance, or on-premise installation if your institution requires local hosting for compliance or data security. Both options provide the same functionality, security, and support.
Implementation depends on the scope and level of customization. A standard, off-the-shelf implementation can take as little as 2 months, while more complex deployments that require integrations or extensive configuration may extend beyond that. Our team follows a structured onboarding and training plan to ensure a smooth transition.
Fibi provides comprehensive onboarding support, including administrator training, user guides, video tutorials, and helpdesk assistance. We also conduct live workshops for faculty and staff to ensure adoption. Ongoing support is included after go-live.
Absolutely. Our implementation team assists with data migration from legacy systems (spreadsheets, in-house tools, or older eRA systems). We ensure that historical grants, protocols, agreements, and disclosures are preserved and accessible in Fibi.
Fibi COI is fully integrated into the broader Fibi ecosystem, enabling seamless coordination across compliance, pre-award, and post-award functions in a unified platform:

Support human subjects research compliance with centralized protocol and committee management.

Facilitate ethical and regulatory oversight of animal research protocols.

Manage the complete grant lifecycle from proposal to closeout.

Centralize and streamline research agreement initiation, review, and tracking.
Manage the complete lifecycle of biosafety protocols in one system.
Experience how Fibi COI can revolutionize conflict of interest management at your institution.