Manage the entire IRB lifecycle with our advanced IRB compliance software, ensuring smooth research
Connect principal investigators, IRB committees, reviewers, and administrators through a shared platform.
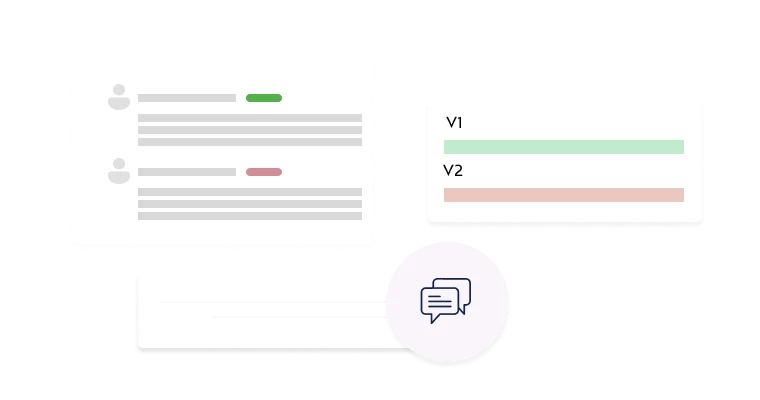
Enable efficient reviews with section-specific commenting, version comparisons, and decision tracking.
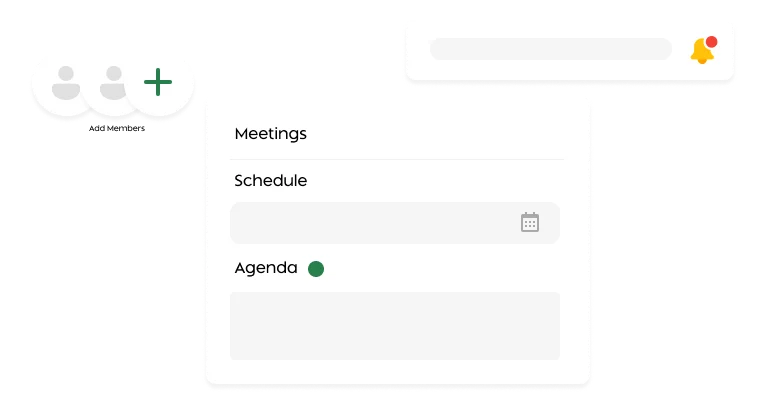
Schedule IRB meetings, auto-generate agendas, capture minutes, and record decisions effortlessly
Customize approval processes to match institution’s needs
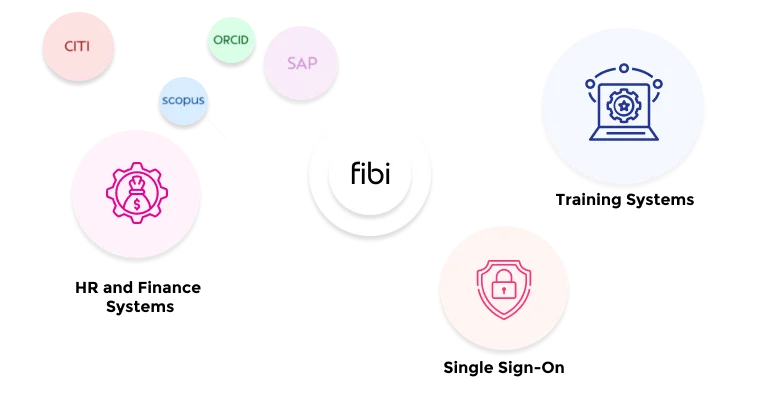
Link IRB functions with Fibi’s pre-award and post-award modules for end-to-end research management.
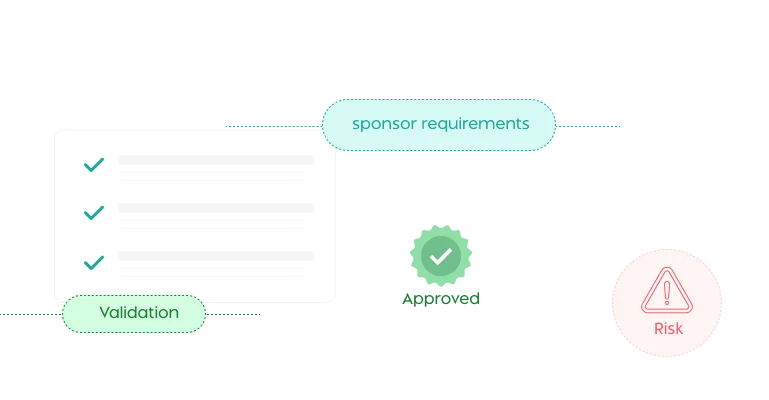
Ensure adherence to regulatory requirements with built-in reminders and notifications
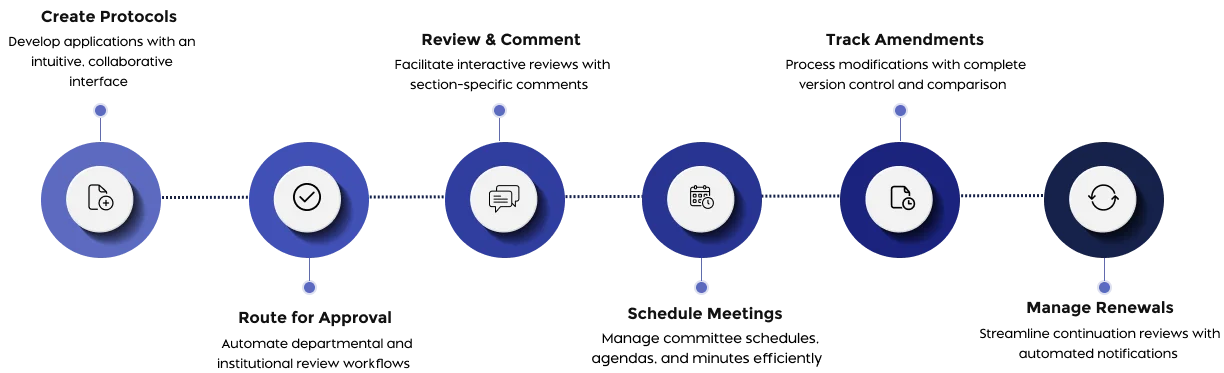
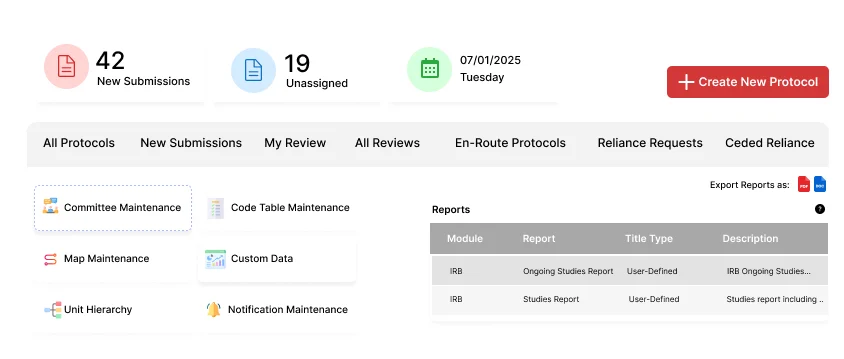
Fibi IRB is an electronic IRB (Institutional Review Board) management system that streamlines human subjects research compliance. It provides a unified platform for all IRB activities: protocol submissions, reviews, committee management, and reporting. By digitizing these processes, Fibi IRB helps institutions enhance transparency, ensure regulatory adherence, and speed up approvals.
Fibi IRB manages the full IRB lifecycle from application to closeout. Investigators can build detailed protocol submissions in a section-based interface, and reviewers have interactive tools to comment and compare versions. The software maintains comprehensive version histories (so you can compare any two submissions side-by-side) and logs every change, ensuring all decisions are documented.
Yes. It includes robust meeting and review management features. IRB administrators can schedule meetings (adhoc and recurring meetings), generate customized agendas from templates, and capture minutes. During review meetings, the system tracks quorum and votes against each protocol, and it supports both single- and multi-site study reviews. Reviewers use a consolidated interface to see entire protocols and add section-specific comments (public or private), enabling efficient deliberations.
Yes. Fibi IRB offers configurable workflows and role-based actions. You can tailor approval paths to match your IRB’s unique process, assign specific actions to PIs, reviewers, or committees, and define who can do what at each step. This flexibility means you don’t have to change your policies to fit the software – Fibi adapts to your existing rules for things like expedited vs. full-board review.
Absolutely. Fibi IRB has built-in compliance tools and automated alerts. It enforces regulatory requirements via configurable checks, sends reminders for renewals/expirations, and prevents lapses in oversight. Dashboards and reports give administrators real-time insight into protocol statuses, approvals, and workflow bottlenecks. In short, it makes it easy to monitor IRB performance and remain audit ready.
Yes. Fibi IRB is part of the fully integrated Fibi suite. It links seamlessly with the Grants, IACUC, and COI modules. For example, an IRB-approved protocol can automatically update related grant or COI records. This end-to-end integration means data (like investigator info) is consistent across compliance, pre-award, and post-award processes.
Yes. Fibi offers flexible deployment options to suit institutional needs. You can choose cloud hosting for scalability and ease of maintenance, or on-premise installation if your institution requires local hosting for compliance or data security. Both options provide the same functionality, security, and support.
Implementation depends on the scope and level of customization. A standard, off-the-shelf implementation can take as little as 2 months, while more complex deployments that require integrations or extensive configuration may extend beyond that. Our team follows a structured onboarding and training plan to ensure a smooth transition.
Fibi provides comprehensive onboarding support, including administrator training, user guides, video tutorials, and helpdesk assistance. We also conduct live workshops for faculty and staff to ensure adoption. Ongoing support is included after go-live.
Absolutely. Our implementation team assists with data migration from legacy systems (spreadsheets, in-house tools, or older eRA systems). We ensure that historical grants, protocols, agreements, and disclosures are preserved and accessible in Fibi.
Fibi IRB integrates seamlessly with the full Fibi research management suite—bringing together compliance, pre-award, and post-award activities in one connected platform:

Manage the complete grant lifecycle from proposal to closeout.

Oversee animal research protocols with built-in compliance and review workflows.

Conflict of interest disclosure and management.

Centralize and streamline research agreement initiation, review, and tracking.
Manage the complete lifecycle of biosafety protocols in one system.
Experience how Fibi IRB can revolutionize research compliance.