Manage the full lifecycle of biosafety protocols within a single, integrated system.

Ensure adherence to NIH Guidelines, CDC regulations, and institutional biosafety standards for laboratory research.
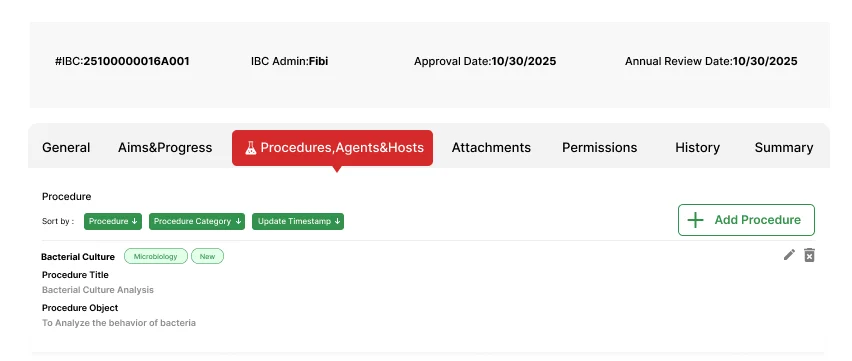
Capture and track biological agents, host organisms, materials, and procedures with precision and traceability.
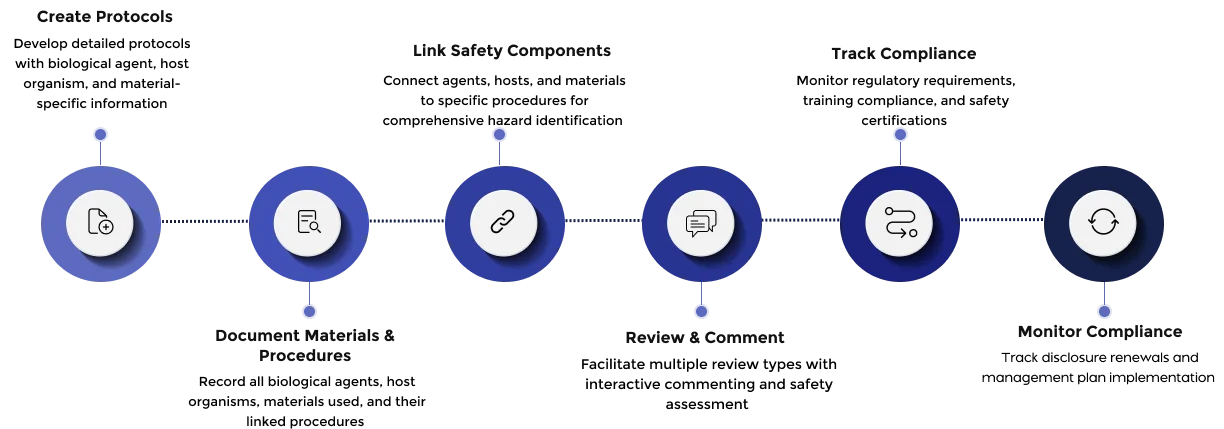
Support all review types from administrative expedited reviews to full committee assessments.
Customize processes for principal investigators, biosafety officers, reviewers, and administrators.
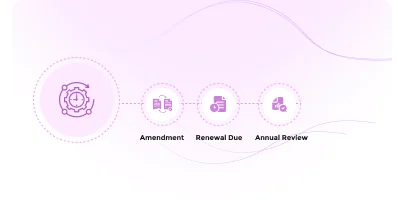
Easily manage protocol amendments, track continuing reviews or renewal requirements and manage adverse events efficiently.
Build detailed protocols with species-specific information

Easily create new submissions based on existing approved protocols

Capture biosafety levels, classifications, and risk assessments for biological agents
Record host species, strains, and relevant biological characteristics
Document both biological materials (cell lines, organisms) and non-biological materials (chemicals, equipment) used in research

Record procedure types, methodologies, and associated safety protocols for each research activity
Connect agents, hosts, and materials to specific procedures for comprehensive hazard assessment

Secure electronic certification with comprehensive audit trail

Covering the entire biosafety oversight lifecycle including amendment processing, annual/continuing review cycles, and adverse event/incident reporting. Outcome-based workflows ensure that each decision (approval, modification request, deferral, or disapproval) triggers the appropriate actions and notifications.

Support administrative, expedited, full committee, and designated reviewer assessments
Add comments, annotations, and attachments during the review process
Compare versions side-by-side to easily identify modifications and safety-related changes

Track all changes across submissions with complete version logging

Document and monitor biosafety risk evaluations and mitigation measures

Streamline annual reviews with automated notifications and compliance reminders
Streamline protocol amendment submission, review, and approval with automated routing, reviewer assignments, and real-time status visibility

Enable fast and compliant reporting of adverse events, serious/unanticipated problems, and incidents with automated notifications, documentation workflows, and review tracking.
Centralized database of all biosafety levels and agent classifications used in protocols
Document specific materials, equipment, and safety measures associated with each procedure

Log laboratory locations, containment levels, and facility designations

Link qualified and trained staff to specific procedures, agents, and host organisms
Support for training compliance and biosafety certification validation
Monitor biological and non-biological materials through their research lifecycle
Create and manage mitigation strategies for identified conflicts.
Schedule reminders for protocol renewals, training expirations, and compliance deadlines
Generate agendas and minutes for biosafety committee meetings
Comprehensive history of all protocol actions, decisions, and compliance status changes

Capture institution-specific biosafety requirements through dynamic questionnaires

Support for streamlined administrative reviews when appropriate
Connect seamlessly with Fibi's research suite for end-to-end oversight

Configure business rules for protocol validation and safety checks

Send targeted notifications to relevant stakeholders and committee members

Generate institution-specific compliance reports for audit and regulatory purposes
Real-time visibility into protocol status, pending actions, and approval milestones
Fibi IRB integrates seamlessly with our complete research management ecosystem, providing unified oversight of
all research activities.
Fibi IRB integrates seamlessly with our complete research management ecosystem, providing unified oversight of all research activities.

Manage the complete grant lifecycle from proposal to closeout.

Centralize and streamline research agreement initiation, review, and tracking.

Support human subjects research compliance with centralized protocol and committee management.

Facilitate ethical and regulatory oversight of animal research protocols.

Manage conflict of interest disclosures and reviews with institution-wide visibility and control.
Experience how Fibi IBC can revolutionize biosafety protocol management at your institution.