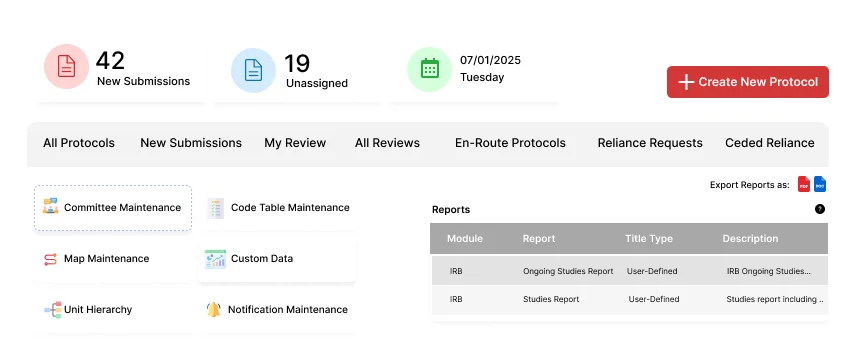
Manage the full lifecycle of animal research protocols within a single, integrated system.
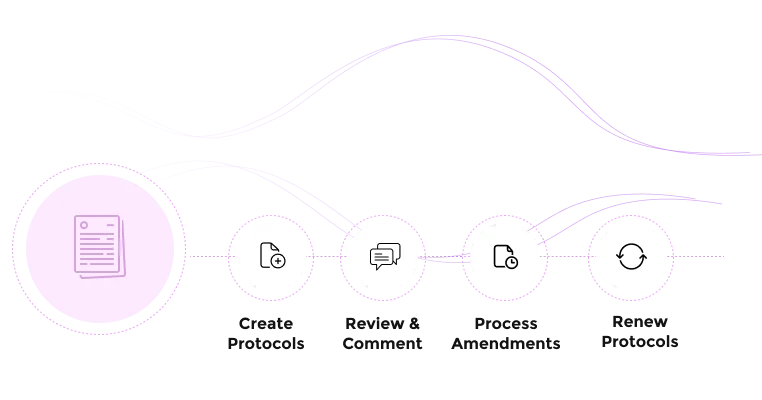
Ensure ethical treatment and regulatory conformity for laboratory animals
Record species, procedures and materials, with precision and traceability.
Support all review types from administrative to full committee
Customize processes for PIs, reviewers, and administrators
Easily manage amendments and track continuing reviews or renewals.
Build detailed protocols with species-specific information.

Easily create new submissions based on existing protocols.

Capture details such as procedure category, type, and pain/distress levels.

Record species names, strains, counts, and USDA categories.
Secure electronic certification with comprehensive audit trails.

Support administrative, full committee, designated member, and veterinary reviews.

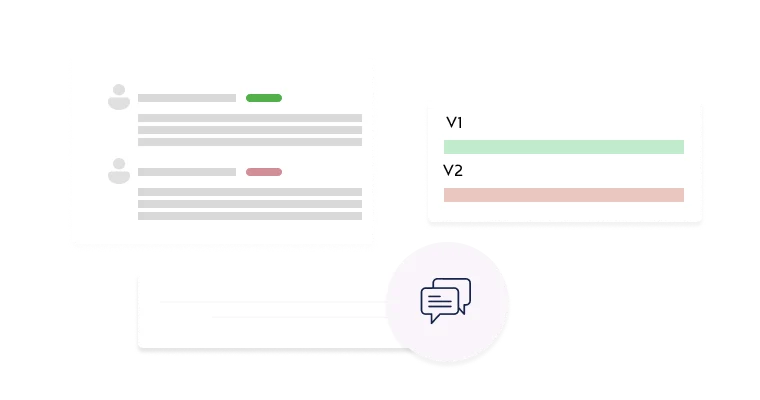
Add comments and attachments during the review process.
Compare versions side-by-side to easily identify modifications.

Track all changes across submissions with complete version logging.

Streamline annual reviews with automated notifications.

Document materials used in procedures.

Log housing and procedural locations.

Link qualified staff to specific procedures and animals.
Support for veterinary consultation and verification.

Generate detailed reports on animals used in research.
Role-specific views for PIs, reviewers, and administrators.
Schedule reminders for renewals, expirations, and training requirements.
Generate agendas and minutes for committee meetings.
Comprehensive history of all protocol actions and decisions.

Capture institution-specific information through dynamic questionnaires.
Connect seamlessly with Fibi’s research suite for end-to-end oversight.
Configure business rules for protocol validation.

Send targeted notifications to relevant stakeholders.

Monitor and validate training requirements for all assigned personnel.

Automated protocol closure processes.
Fibi IACUC is an electronic management system for Institutional Animal Care & Use Committees. It oversees the entire lifecycle of animal research protocols in one platform, helping institutions maintain ethical, compliant animal research. Key functions include protocol development, review workflows, approval, and tracking of all animal use records.
The system captures comprehensive protocol details and enforces humane research standards. Researchers can build protocols with species, strain, and procedure information, while administrators track things like animal counts, pain/distress levels, etc. Fibi IACUC ensures every procedure is documented: it logs materials used, procedure types, and tracks animal-specific data for full traceability.
Yes. Fibi accommodates all review types (administrative review, full committee, designated member, veterinary review, etc.). It also streamlines amendments and continuing reviews: any protocol renewal or change request triggers automated workflows and notifications. In practice, this means reminders are sent when IACUC approvals are about to expire, and reviewers can easily process modifications in the same system.
Yes. It provides role-based workflows so that tasks are assigned appropriately to PIs, veterinarians, and committee members. For example, some protocols may require veterinary sign-off, while others go directly to a committee. Fibi lets you define these paths so that each protocol follows the correct approval route without manual intervention.
Absolutely. Fibi IACUC is fully integrated with other Fibi modules. Animal research protocols can be linked to related grants or compliance activities, and investigator data stays consistent across IRB and COI modules. This unified approach simplifies reporting on overall research compliance and avoids duplicate data entry across systems.
Yes. Fibi offers flexible deployment options to suit institutional needs. You can choose cloud hosting for scalability and ease of maintenance, or on-premise installation if your institution requires local hosting for compliance or data security. Both options provide the same functionality, security, and support.
Implementation depends on the scope and level of customization. A standard, off-the-shelf implementation can take as little as 2 months, while more complex deployments that require integrations or extensive configuration may extend beyond that. Our team follows a structured onboarding and training plan to ensure a smooth transition.
Fibi provides comprehensive onboarding support, including administrator training, user guides, video tutorials, and helpdesk assistance. We also conduct live workshops for faculty and staff to ensure adoption. Ongoing support is included after go-live.
Absolutely. Our implementation team assists with data migration from legacy systems (spreadsheets, in-house tools, or older eRA systems). We ensure that historical grants, protocols, agreements, and disclosures are preserved and accessible in Fibi.
Fibi IACUC integrates seamlessly with the broader Fibi platform, unifying compliance, pre-award, and post-award
activities across your institution:
Fibi IACUC integrates seamlessly with the broader Fibi platform, unifying compliance, pre-award, and post-award activities across your institution:

Centralize and streamline research agreement initiation, review, and tracking.

Support human subjects research compliance with centralized protocol and committee management.

Manage the complete grant lifecycle from proposal to closeout.

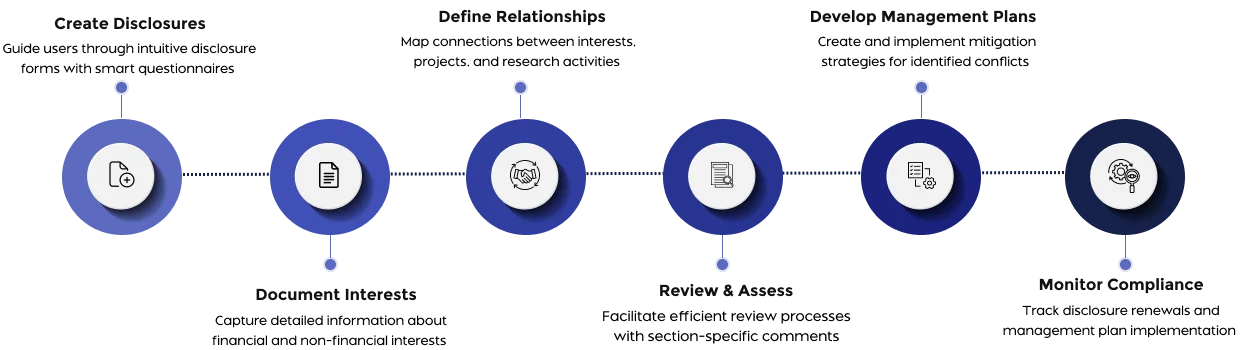
Manage conflict of interest disclosures and reviews with institution-wide visibility and control.
Manage the complete lifecycle of biosafety protocols in one system.
Experience how Fibi IACUC can revolutionize animal research compliance at your institution.